Electrons flow when cell produces energy wire graphite electrode zinc electrode. A dry cell is a type of electric battery commonly used for portable electrical devices.

Structure Types And Working Of Dry Cell Utmel
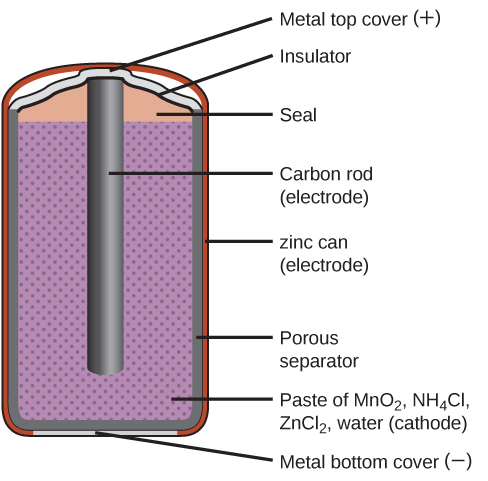
Cathode is an inert graphite rod in the center of the cell immersed in an electrolyte paste.

. A primary cell is not rechargeable. Arachaeal flagella grow by adding subunits at the basenot the tipof the flagella. A dry-cell battery does not contain this and more diminutive dry-cell batteries such as alkaline or lithium-ion are typically used in transferable electronics such as toys phones and laptops.
The solid or gel-like substance that surrounds a typical cell. Set of membranous discs in the cytoplasm usually between the ER and plasma membrane of the cell. A LeClanche dry cell has Zinc oxidized and NH4 being reduced.
Dry cell consist on two mettalic plates and electrolyte. Produces small membranous spheres called vesicles such as lysosomes. The cell is made up of an outer zinc container which acts as the anode.
Proteins with carbohydrate groups attached. Electrodes are typically good electric conductors but they need not be metals. Starch is added to the paste make it thick so that it cannot be leaked out.
Placing two batteries in parallel. Cells are connected in series in order to A. A standard dry cell comprises a zinc anode usually in the form of a cylindrical pot with a carbon cathode in the form of a central rod.
Arachaeal flagella are 10-14 nm in diameter. I hope this helps. This is the positive electrode which is surrounded by the electrolyte typically a paste of ammonium chloride and manganeseIV oxide all of which are in a zinc container which is the negative electrode.
The electrolyte is ammonium chloride in the form of a paste next to the zinc anode. In an electrochemical cell reduction and oxidation reactions take place at the electrodes. In plant cells organelles called _____ contain chlorophyll in the thylakoid membrane and are the sites of photosynthesis.
Electrolyte is moist paste of ammonium chloride and zinc chloride. This is the positive electrode which is surrounded by the electrolyte typically a paste of ammonium chloride and manganeseIV oxide all of which are in a zinc container which is the negative electrode. Typical dry cell batteries contain a zinc anode and a carbon cathode and produce a potential difference of 15 V.
The following statement about the measurement of electrical quantities is correct. Dry cell is compact form of Leclanche cell. The dry cell battery is one of the most commonly used types including AA 9-volt and watch batteries.
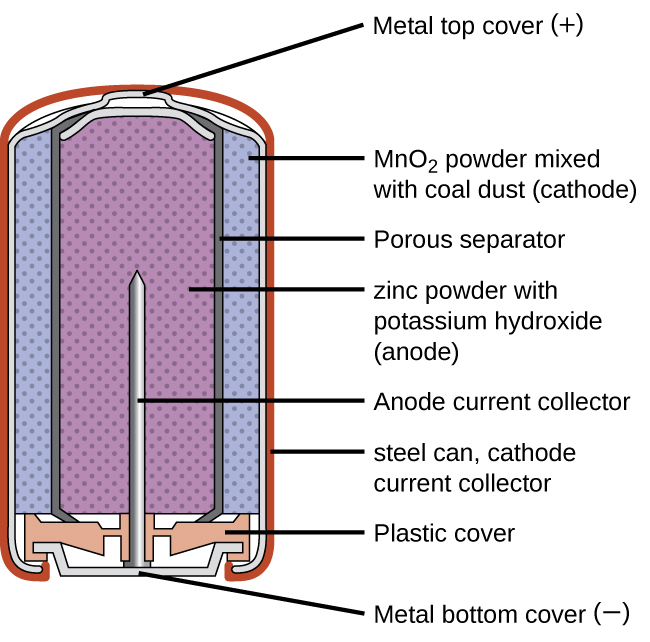
Generally the metal container will be zinc whose base acts as a negative electrode anode and a carbon road acts as a positive electrode cathode. A common dry cell is a zinc-carbon battery sometimes called the dry Leclanché cell with a nominal voltage of 15 volts same as the. Doubling the quantity of Zns II.
A single layer of cells whose length breadth and depth are about the same size. The in between space is filled with a paste of and. Arachaeal flagella rotate from the flow of hydrogen ions across a membrane.
This traditional dry cell consists of a carbon-rod cathode positive terminal immersed in a moist paste of Mn IV O 2 Zn II Cl 2 NH 4 Cl and powdered carbon which is contained in a metallic zinc-can anode negative terminal. A dry cell consists of a metal container in which a low moisture electrolyte paste covers the graphite rod or a metal electrode. A graphite rod which acts as cathode is placed in the center and is coated with powdered and carbon.
The proteins of archeal flagella differ from those in bacterial flagella. Dry cell changes chemical energy into electrical enery by the means of chemical reaction. The constant addition of new cell membrane is typically matched by a constant recycling of the cell membrane which maintains the size of the.
A chemical reaction within the battery creates an electrical charge that. To get from the nucleus to the cytoplasm RNA must pass through----- in the nuclear membrane. The cathode is a central carbon rod surrounded by a mixture of.
Alkaline dry cells offer improved performance because they are secondary cells. Batteries contain a variety of electrodes depending on the battery type. A common dry cell is the zinccarbon cell sometimes called the dry Leclanché cell with a nominal voltage of 15 volts the same as the alkaline cell since both use the same zincmanganese dioxide combination.
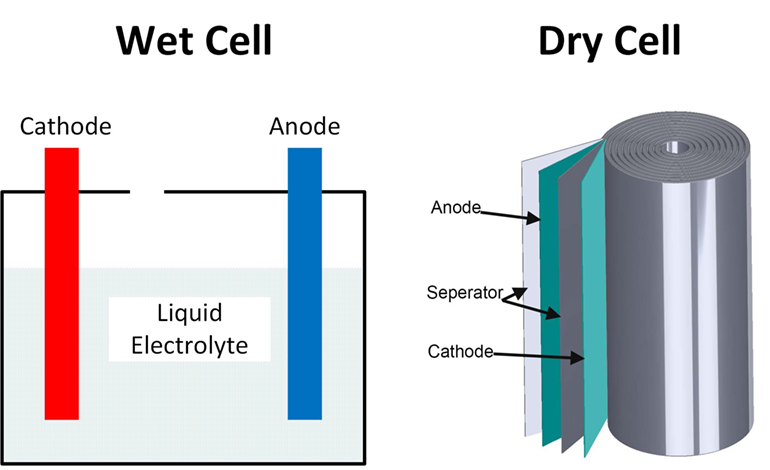
Wet cell batteries carry a liquid electrolyte such as sulfuric acid - a hazardous corrosive liquid. The voltage of an alkaline dry cell is approximately 15. RNA carries information for protein synthesis from DNA in the nucleus to the ribosomes in the cytoplasm.
Unlike a wet cell a dry cell can operate in any orientation without spilling as it contains no free liquid making it suitable for portable equipment. A standard dry cell comprises a zinc anode usually in the form of a cylindrical pot with a carbon cathode in the form of a central rod. Lead-acid batteries are based on lead electrodes.
A single layer of cells longer than they are wide. A dry cell uses a paste electrolyte with only enough moisture to allow current to flow. Dry cells are not really dry but contain a moist mixture.
A dry cell battery has a central rod usually made of graphite. A cell contains a large number of ribosomes would produce a large number of----- molecules. Resistance is measured in units called ohms.
A common dry-cell battery is the zinc-carbon battery which uses a cell that is sometimes called the Leclanché cell. It consists of a cylindrical zinc container which acts as anode. Several layers of cells but without a basement membrane.
A parallel circuit contains two or more electrical paths for current to follow. Several layers of cells all of the same type. Given that many electronic devices require additional voltage which of the following would result in an overall increase in voltage.
A typical small dry cell battery has a voltage of A. Which of the following would be found in the membrane of an animal cell. D A dry cell battery has a central rod usually made of graphite.
Nuclear Pore or Nuclear envelope. Correct options are C and D Anode is zinc. Dry cell batteries are different from wet cells because their electrolytes are contained in a low-moisture paste while a wet cell has electrolytes contained in a liquid hence the difference in names.
17 5 Batteries And Fuel Cells Chemistry
17 5 Batteries And Fuel Cells Chemistry

0 Comments